
So, all the pairs get arranged to minimize the repulsion. The valence shell electron pair repulsion theory is as follows:Įlectron pair is the number of electron pairs present around the central atom in a molecule.Īccording to VSEPR the electron pairs present around the central atom repel each other.

Then we will arrange the remaining electrons around each atom to complete the octet. Then we will subtract the electrons used in bond formation from the total valance electrons. So, we will count the total electron used in bond formation. Two electrons are used in the formation of a bond. Then we will count total valence electrons. The least electronegative atom is the central atom. Then we will decide the central atom around which we will write all atoms of the molecule. As some atoms join and form covalent bonds between them within a molecule. Then by using valence shell electron pair repulsion theory the geometry can be determined.Ĭomplete step-by-step answer :We will write the Lewis structure as follows:įirst we will write the basic structure. structure ClO4 is one of the most important and interesting chapters of chemistry. We can draw the Lewis structure of the molecules to determine the electron pair of the central atom.
#Clo2 molecular geometry how to
As a result, the compound is a peroxide, but more specifically referred to as barium peroxide.Hint: To determine the shape of a molecule we should know how to write the Lewis structure and about VSEPR theory. Barium has an oxidation state of +2 so the oxygen atoms have oxidation state of -1. Is peroxide a BaO2?Īnswer: BaO2 is a peroxide. It is an intermediate in the oxidation of sodium by oxygen. This yellow-orange solid is a salt of the superoxide anion. Sodium superoxide is the inorganic compound with the formula NaO2. What is magnetic character of anion of KO2?Īnswer: What is the magnetic character of the anion of KO2 ? Solution : Anion of KO2 =O-2 (superoxide ion) which has one unpaired electron and hence in paramagnetic. … Hence, KO2 behaves as paramagnetic molecule.
#Clo2 molecular geometry free
So, in KO2 the oxygen atoms bear -1/2 oxidation state and they also behave as a free radical species, having an unpaired electron. KO2 is a superoxide in which, only one electron is released from the dioxygen atom and a superoxide ion is represented as O2. Is in a molecular orbital, there is an existence of unpaired electrons, the molecule formed is referred to as paramagnetic in nature. Is Na2O2 diamagnetic or paramagnetic?Īnswer: Na2O2 is diamagnetic in nature since it does not have unpaired electrons in the molecular orbital. Therefore, all subshells must be completely filled. In NO+ , due to loss of 1 electron, the no. KO2 is paramagnetic because of the presence of unpaired electrons. … Which is more unpaired electrons in orbitals then that will show paramagnetic, which is very less unpaired or fully fiilled orbital that is diamagnetic. Why is ClO2 paramagnetic?ĬlO2 is paramagnetic as it has odd number of electrons. – Cl2O7 has no unpaired electron in it, so it is diamagnetic in nature.ĬlO2 is paramagnetic due to unpaired electron(s). The carbon in cyanide is the central atom.
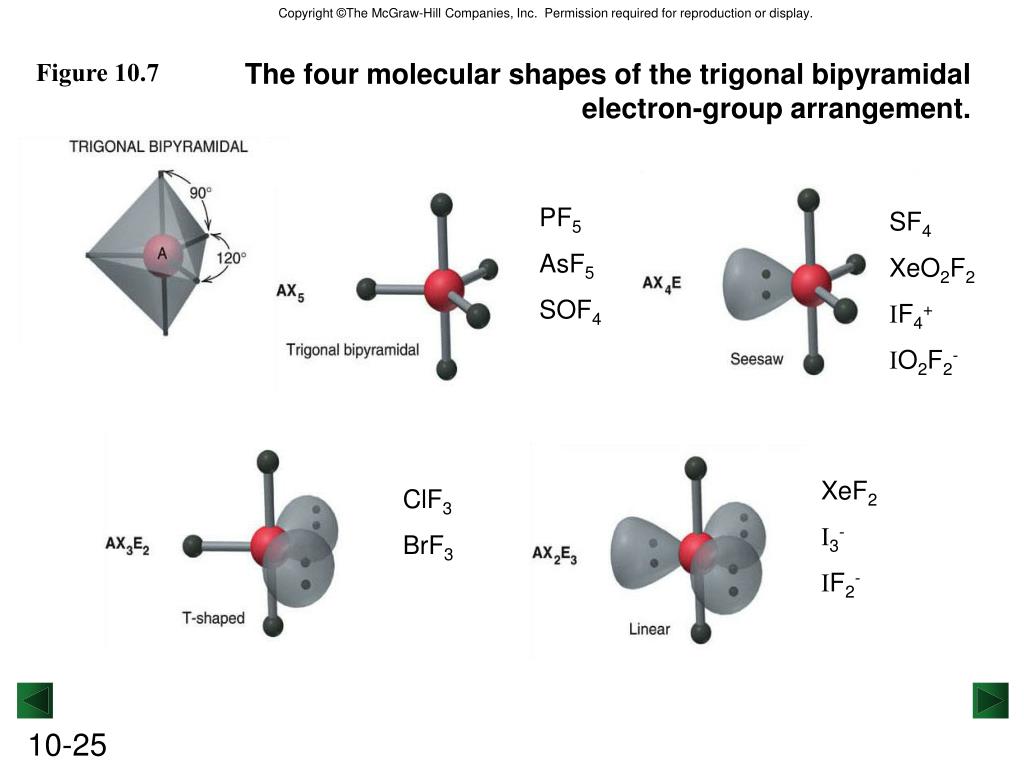
A covalent bond is present in the molecule. LinearThe molecular geometry of hydrogen cyanide is linear. Is ClO2 polar?Ī) Structure of ClO−2 C l O 2 − is: It has a bent structure where the 2 polar O-Cl bonds and 2 lone pair of electrons do not cancel out… What is the molecular shape of HCN? How do you draw clo2? Do clo2 and clo3 have the same molecular geometry? Is ClO2 paramagnetic or diamagnetic?ĬlO2 is paramagnetic due to unpaired electron(s). Two electrons are used in the formation of a bond.…Shape of ClO−2 ion is: A.
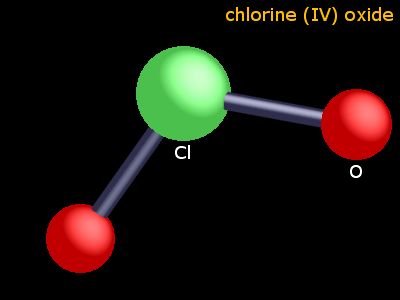
That means that the Chlorine (Cl) atom will have an unpaired electron and therefore wont have an octet (it. Brockway, a graduate student of Linus Pauling, proposed a structure that involved a three-electron bond and two single bonds. ClO2 has an odd number of valence electrons (19). The molecule ClO2 has an odd number of valence electrons, and therefore, it is a paramagnetic radical. It is a reddish to yellowish-green gas at room temperature that dissolves in water.
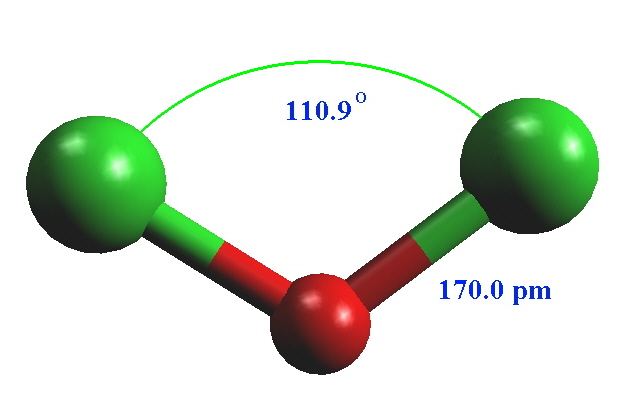
What does ClO2 look like?Ĭhlorine dioxide (ClO2) is a chemical compound consisting of one chlorine atom and two oxygen atoms. The molecular geometry of ClO2- is V-shaped and electron geometry is tetrahedral. The bond angle in ClO2- is slightly less than 109°. The overall formal charge in ClO2- is -1. Is ClO2 tetrahedral?ĬlO2- is a polar molecule due to the asymmetrical distribution of charges caused by the presence of lone pair electrons. There is zero lone-pair around thr central atom(P) so the geometry is tetrahedral. The electron pair geometry around POCl3 is tetrahedral. What is the the shape molecular geometry of POCl3? ClO2 is bent in shape with tetrahedral spatial geometry having two pairs of lone pair.


 0 kommentar(er)
0 kommentar(er)
